Order of a Reaction
Order of a Reaction: Overview
This Topic covers sub-topics such as First Order Reactions, Zero Order Reactions, Order of a Reaction, Second Order Reactions, Rate Determining Step, Elementary Reactions, Complex Reactions and, Formation of Intermediate during a Chemical Reaction
Important Questions on Order of a Reaction
The half-life for decay of radioactive is 5730 years. An archaeological artifact containing wood has only 80% of the activity as found in living trees. The age of the artifact would be:
[Given: log 1.25 = 0.0969]
For a reaction, in an aqueous medium, the rate of reaction is given by
The role of is.
Which of the following is incorrect?
For the following elementary reaction, determine its order of reaction and the dimensions of the rate constant:
For a reaction, , the rate is given by , hence, the order of the reaction is:
For the reaction product
order of reaction is :-
If in a unimolecular reaction, takes place according to the mechanism
I.
II.
where are the rate constants and P, and stand for product molecule, normal molecules of reactants and activated molecules of reactants respectively.
Which of the following statements are correct?
For next two question please follow the same
If in a unimolecular reaction, products takes place according to the mechanism
I.
II.
where are the rate constants and P, and stand for product molecule, normal molecules of reactants and activated molecules of reactants respectively.
Which of the following expressions are correct?
The rate of the first order reaction products is , when reactant's concentration is . The rate constant for the reaction will be
The reaction between and occurs in the following steps:
The reaction intermediate in the reaction is
The slope of the straight line obtained by plotting rate versus concentration of reactant for a first order reaction is.
The unit of rate constant for first order reaction is
Statement : The exponents for concentration do not necessarily match the stoichiometry coefficients in the rate law expression.
Statement : In the rate law expression, exponents for concentration are determined by experiments not by balanced chemical reaction.
is a first order reaction.
If the initial pressure is and the total pressure at time 't' is , then the rate constant is:
Find out the order for the given reaction
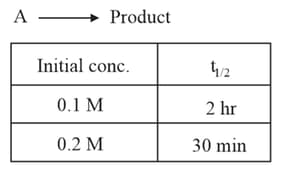
Calculate the half life for a zero order reaction, if reaction is completed in .
What is the correct representation of graph for a reaction of first order kinetics (A: moles of reagent remaining, t: time)?
Identify the correct potential energy vs reaction co-ordinate graph, which is consistent with given mechanism.
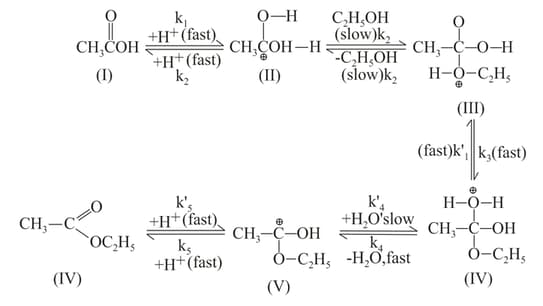
The mechanism of esterification in presence of acid catalyst (H2SO4) is proposed as follows:
Radioactive decay is an example of which order of reaction?
Calculate the overall order of the reaction, given that a reaction involving A, B and C as reactants is found to obey the rate law, rate=. When the concentration of A, B and C are doubled separately, the rate is also found to increase two, zero and four times respectively.
